KOREAN ENGLISH
주요취급품목
PolyWAX LPTM
PolyGLYCOPLEX ATM
PolyHYDROXYETHYL ATM
SDS Removal
PolyMETHYL ATM
PolyPROPYL ATM
TopTipsTM/Nu TipsTM
분취용 HPLC컬럼
Flash Cartridges
Flash LC 시스템
Detectors
Chromatography Gels
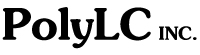
Proteomics
SCX-RPC of
Tryptic Digests: No single analytical method suffices
to identify every protein in a very large collection. A widely-used approach
("shotgun" or "bottom-up" proteomics) is to digest all the
proteins in a mixture with trypsin and divide the resulting peptides into sets
small enough to permit identification via MS/MS or QTOF-MS. For samples with
< 200 peptides, reversed-phase HPLC usually provides adequate resolution,
but larger samples must first be divided into subsets via a complementary
method. The best way to accomplish this is to use Strong Cation-Exchange
(SCX) to separate the digest into fractions by differences in
charge-to-mass ratio. Each SCX fraction can then be analyzed separately via
RPC-MS. This sequence has been used to identify as many as 12,000 peptides in a
sample. Our PolySULFOETHYL Aspartamide™ material is the best for the
purpose and is used by most proteomics labs. It is best performed with a salt
gradient at pH 2.7-3.0, where carboxyl- groups have lost their negative charge
and nearly all peptides have a net + charge. About 65% of the peptides in a
typical complex tryptic digest have a net charge of +2 (due to the basic
N-terminus and the Lys- or Arg- at the C-terminus). A linear salt gradient
would cause these to elute bunched up in a few fractions, defeating the purpose
of a second dimension of chromatography. Instead, elute the column with a
2-segment linear gradient; a shallow gradient (to about 180 mM salt) over most
of the gradient time to spread out the +1 and +2 peptides and a steep gradient
to ~ 0.5 M salt to elute the +3 and +4 peptides. The following is a good
example of uniform distribution of the peptides in a tryptic digest.
Order PolySULFOETHYL A™ now.
Online vs.
Offline SCX Fractionation: Packed capillaries of PolySULFOETHYL
A™ can be eluted via steps of increasing salt concentration, with each
fraction going to a desalting RPC trap cartridge or directly to a RPC
capillary. This arrangement is commonly called MuDPIT. The alternative
is to collect fractions offline and then inject them separately onto the RPC
capillary ("divorced MuDPIT").
Online advantages: Easier to handle extremely small samples and to automate.
Offline advantages:
1) Can be performed with simpler equipment.
2) The mobile phase can contain 20-25% ACN, which can be removed prior to the
RPC step. This affords sharper peaks. Result: A higher percentage of the
peptides elute within a single collected fraction instead of being split
between two adjacent fractions (in favorable cases, >75% of the total
peptides in a really complex tryptic digest). This increases success in peptide
identification, since you are more likely to get a detectable quantity (~ 15
fmol) of a low-abundance peptide within a single fraction and it is less likely
to be sharing the fraction with a peptide from a high-abundance protein (which
could suppress its ionization at the MS step).
3) The SCX column can be larger than the RPC capillary. This permits loading of
more sample, making it more likely that you will have > 15 fmol of each
peptide to detect. It also permits use of a faster flow rate. This facilitates
collection of numerous fractions (as many as 100 at some labs). That, in turn,
increases the chances of identifying the peptides of low abundance, since it is
less likely that they will be sharing a fraction with a peptide of high
abundance. For example, Gan et al. (Proteomics 5 (2005) 2468) identified
2.8x more proteins by collecting 40 SCX fractions than they did with 25 SCX
fractions.
4) It is possible to use linear gradients instead of step gradients. This
increases resolution.
CONCLUSION:
Offline fraction collection is superior . Kevin
Blackburn (NC St. U) states* that he identifies 3x more peptides with offline
fraction collection than with online, with all of the increase representing
peptides of low abundance.
*(ASMS '00 and '01).
Predigest
Fractionation of Intact Proteins : This can be very useful in several cases:
1) There are so many proteins in the sample that even the 2-D combination of
SCX-RPC does not suffice to fractionate the tryptic digest sufficiently to
identify low-abundance peptides.
2) The sample contains a few proteins of much higher abundance than the others.
Collecting them in their own fractions will preclude their tryptic fragments
masking peptides from proteins of low abundance. See the example below of THP-1
monocytes.
3) While the number of proteins is limited, the ones of interest are of low
abundance and would be masked if not separated from the high-abundance proteins
prior to digestion. See examples of this with Histones H4 and H1.5.
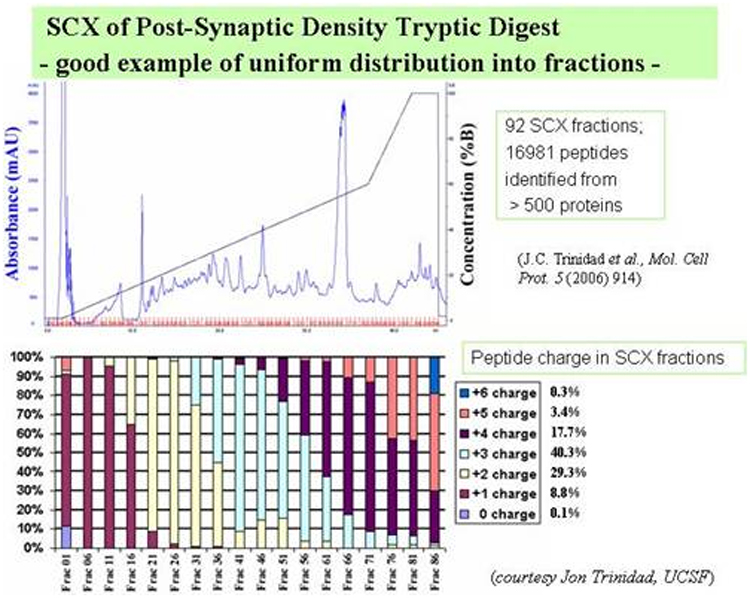
4) Distributing proteins into fractions prior to digestion increases the
chances of identifying a specific protein by more than one peptide. See the
following schematic:
General-purpose
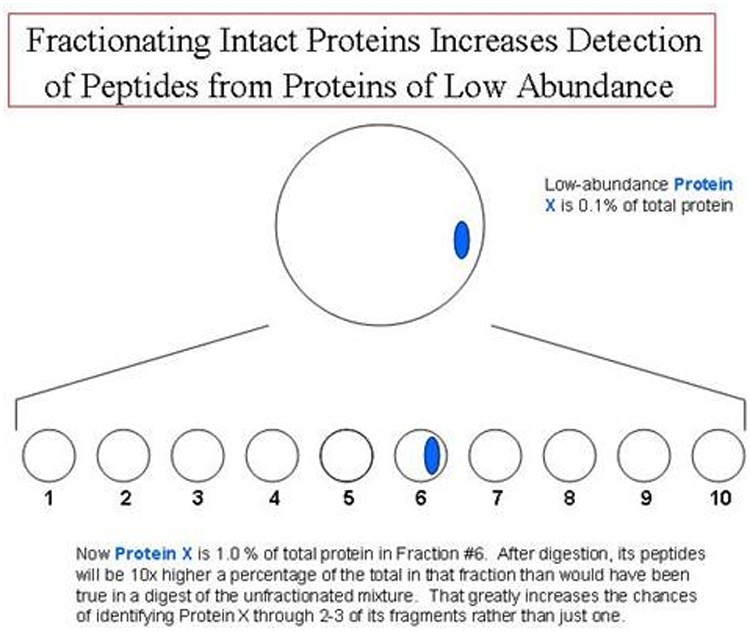
methods for this include Hydrophobic Interaction (HIC) and ion-exchange (IEX)
chromatography. HIC is a good method for fractionating water-soluble proteins
by differences in their hydrophobicity [BELOW]. Unlike reversed-phase
chromatography, it is a nondenaturing mode. However, hydrophobic proteins would
be difficult to keep in solution in HIC.
By contrast,
IEX can be performed with organic solvents in the mobile phase, making it
prospectively compatible with all proteins. See Ion Exchange of Proteins with Organic Solvents.
Using an anion- and a cation-exchange column in series yields a mixed-bed arrangement that retains all proteins. The example below is a crude lysate of THP-1 monocyte cell pellet, obtained with a PolyCAT A™ and a PolyWAX LP™ column. Gradients with volatile salts are possible.
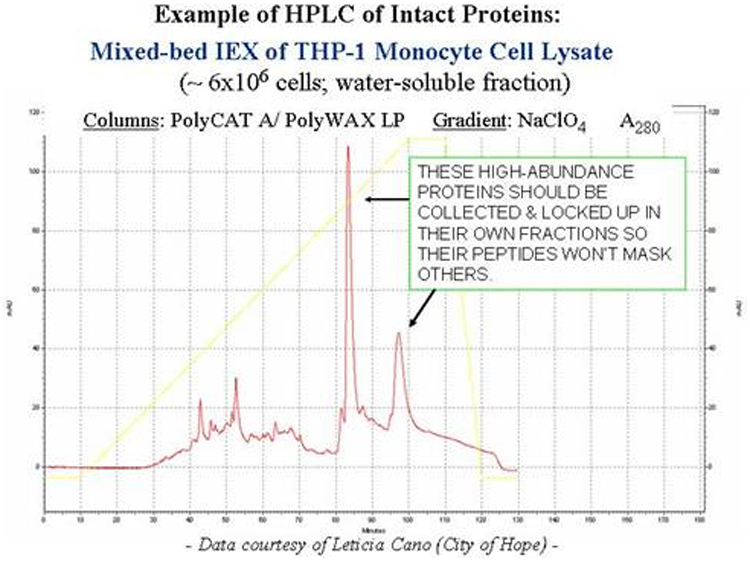
HILIC of
membrane proteins: HILIC works well for membrane
proteins, as per the examples below. Volatile solvents can sometimes be used
for this. Histones are best resolved with PolyCAT A™ columns in the HILIC mode.
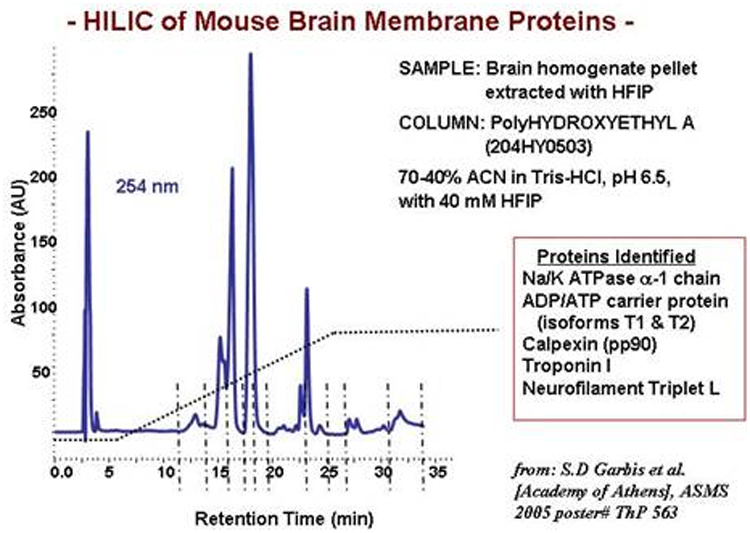
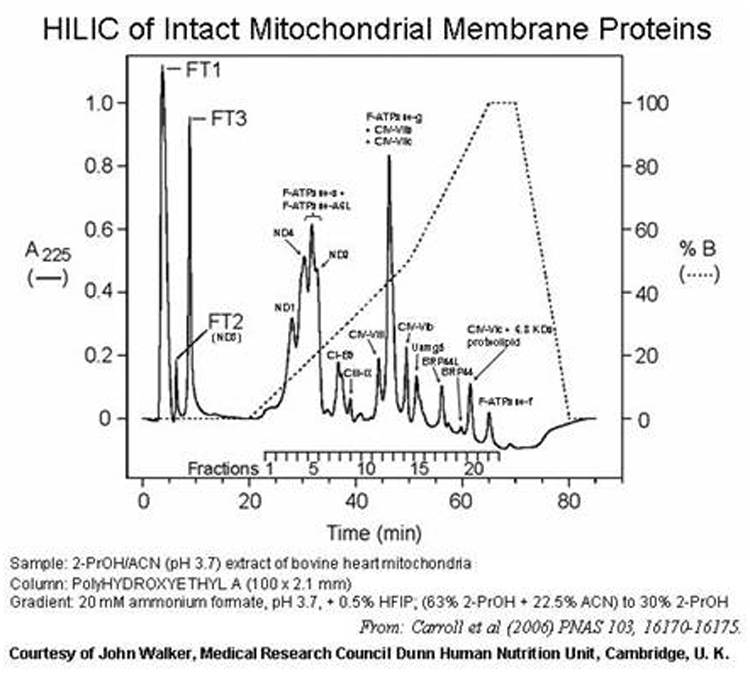
Isolation of Phosphopeptides
and Other Classes of Peptides from Tryptic Digests: The
typical tryptic peptide has a net charge of +2 at pH 2.7-3.0, due to the
N-terminus and the Lys or Arg residue at the C-terminus. Attachment of a
phosphate group lowers the net charge of the peptide to +1. Thus, the
earliest-eluting SCX fractions are enriched in phosphopeptides, as well as
C-terminal and blocked N-terminal fragments. The 200-Å pore version of PolySULFOETHYL
A™ has a higher surface area than the 300-Å material normally used for
proteomics and can pull the +2 peptides away from the +1 peptides reasonably
completely [BELOW]. Beausoleil et al. (PNAS 101 (2004) 12130) have used
this approach to identity over 2000 phosphopeptides from the tryptic digest of
HeLa cell lysate. In various studies, about 20-30% of the phosphopeptides in a
really complex tryptic digest eluted in this +1 window. A good recent
examination of this approach is: J.C. Trinidad et al., Mol. Cell. Proteomics
5 (2006) 914.
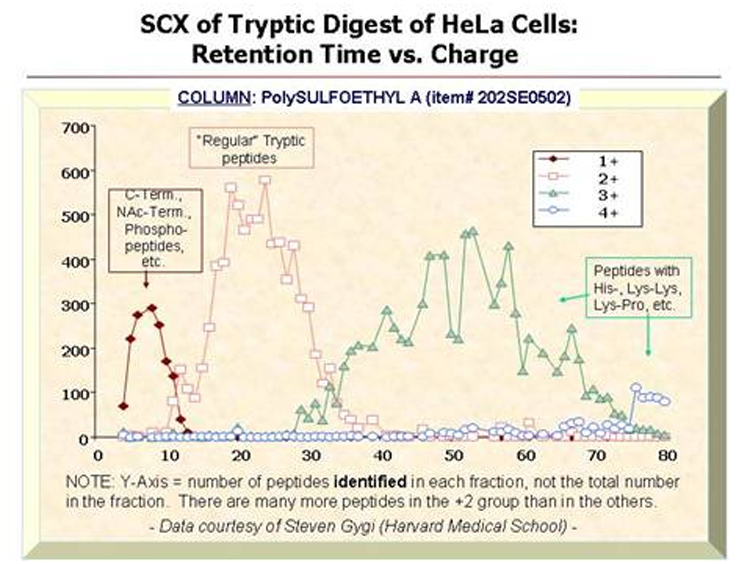
At the other
extreme, crosslinking two +2 peptides results in a peptide with a net charge of
+4. Thus, in a nonreduced tryptic digest, the disulfide-linked peptides elute
appreciably later than most of the other peptides. This method has been used to
isolate them selectively.
Problems with Membranes and Whole Cell Pellets:
1) Dissolving the sample.
2) Eliminating lipids from the resulting solution.
3) Maintaining membrane and structural proteins in solution during subsequent
fractionation.
Such samples
can be dissolved within minutes, at room temp., with a 4:1 solution of HFIP [neat]
and conc. formic acid (96-98%). Membrane proteins can also be extracted with
propanol or acetonitrile containing HFIP (ref.: J. Carroll et al., PNAS 103
(2006) 16170). These solvents avoid the interference that detergents impose
with downstream analytical methods. The lipids in the resulting solution can be
eliminated by passing it through an SPE cartridge in the HILIC mode; e.g.,
item# SPEHY1203, TT200HEA, etc. Lipids (and salts and detergents) are not
retained but proteins and peptides are. The proteins and peptides can then be
eluted with steps to increasingly aqueous solution. To maintain the proteins in
solution during IEX-HPLC, use NaClO4 for the gradient and include
the following in the mobile phases: 50 mM HFIP, 20% ACN and 20% PrOH. In
extreme cases, use 35% ACN and 35% PrOH. Alternatively, use PolyHYDROXYETHYL
A™ in the HILIC mode, per the examples above with membrane proteins.
Order PolyHYDROXYETHYL A™ now.
ICAT®; iTRAQ®;
MuDPIT :
ICAT: If you can ascertain a protein's presence by identification of 2 or 3 of
its tryptic fragments, then identification of the rest is redundant. The
"AT" part of ICAT eliminates most of the tryptic fragments from a
protein, making it possible to identify more proteins from complex mixtures.
The complete digest is usually fractionated by SCX on PolySULFOETHYL A™
prior to affinity purification on an avidin column. This cleans up the sample
and eliminates excess ICAT reagent, which might otherwise saturate the binding
capacity of the avidin.
iTRAQ: This facilitates measurements of relative abundance but does not
necessarily simplify the mixture in the manner of ICAT. After reaction with the
iTRAQ reagents, be sure to drop the pH to 3.0 or less and desalt the mixture in
order to get good retention in SCX.
MuDPIT: See discussion above about online vs. offline methods.
Common Pitfalls
in Proteomics Strategies :
1) Failure to eliminate lipids: These clog RPC columns and interfere in
general. Eliminate them with a SPE-HILIC cartridge or else perform ion-exchange
with a mobile phase containing > 40% organic solvent.
2) Using urea, SDS or Gd.HCl during trypsinization: These are incompatible with
RPC and/or MS. Use 30% PrOH instead. Ref: W.K. Russell et al., Anal. Chem.
73 (2001) 2682.
3) Failure to adjust pH after trypsinization or iTRAQ reaction: Trypsinization
and iTRAC derivatization is performed ~ pH 8 while subsequent SCX is performed
at pH 2.7-3.0. Failure to adjust the pH will lead to elution of a sample in the
void volume. If possible, use NH4HCO3 as the
trypsinization buffer instead of Tris, since it can be eliminated via
lyophilization.
4) Sample too salty: If using a SpeedVac® to get rid of NH4HCO3,
take the sample to dryness three times in succession. Desalting iTRAQ reaction
products is better than merely diluting them with the SCX mobile phase.
5) Use of TFA or HFBA: Some authors have recommended their use in the sample
solvent or the starting mobile phase for SCX. DON'T! Tryptic peptides are frequently
not retained with such solvents. Use either 5 mM KH2PO4
or else 0.1% formic acid or acetic acid.
6) Overloading the SCX column or capillary: Recommended maximum loads of
peptide per injection are as follows for PolySULFOETHYL A™ columns:
0.30-mm i.d., 25µg; 1.0-mm i.d., 250µg; 2.1-mm i.d., 1.0 mg; 4.6-mm i.d., 5 mg.
Some groups exceed these levels by up to 6x. This saves labor and yields more
concentrated fractions. Problems:
a) The columns
usually last 1/6 th as long.
b) Peaks are broader. This means there will be more peptides in each collected
fraction. The RPC capillary might be overloaded, resulting in a smaller
percentage of peptides being identified.
CONCLUSION: Use a SCX column large enough for your sample.
PolySULFOETHYL
A™ , PolyCAT A™, PolyWAX LP™, and PolyHYDROXYETHYL A™ are trademarks of PolyLC
Inc. All Rights Reserved.
SpeedVac® is a trademark of Savant Corp.
*ICAT® and
iTRAQ® are trademarks of Applied Biosystems Inc.,
a branch of Applera Corp.
주소
서울특별시 송파구 충미로 5 송파한화오벨리스크 C동 415호
연락처
전화번호 : 02-3012-9003 팩스번호 : 02-3012-9010
intertech9@naver.com
사업자등록번호
215-87-83507 대표이사 : 이홍근
