KOREAN ENGLISH
주요취급품목
PolyWAX LPTM
PolyGLYCOPLEX ATM
PolyHYDROXYETHYL ATM
SDS Removal
PolyMETHYL ATM
PolyPROPYL ATM
TopTipsTM/Nu TipsTM
분취용 HPLC컬럼
Flash Cartridges
Flash LC 시스템
Detectors
Chromatography Gels
PolyCAT A™ Columns
Initial Use: PolyCAT A™ is a
silica-based material with a bonded coating of polyaspartic acid. It is a weak
cation-exchange(WCX) material. Columns are shipped in methanol. Flush new
columns with at least 15 column volumes of water (30 ml for a 200x 4.6-mm),
then condition with a salt solution prior to initial use. A good conditioning
solution is 40 mM EDTA.2Na (filtered, butpH not adjusted) at a low flow rate
for 20-24 hours.
New HPLC columns sometimes absorb small quantities of
proteins or phosphorylated peptides in a nonspecific manner. The sintered metal
frits have been implicated in this. Eluting the column for 20-24 hr. at a low
flow rate with 40mM EDTA.2Na usually solves the problem. This passivates all
metal surfaces in the HPLC system, as well as the column [CAUTION: This
treatment can affect the integrity of the frits in some cases, and should
probably be avoided with columns packed with 3-μm material. In some cases this
has also caused the collapse of 5-μm, 200-Å column packings]. Alternatively,
after flushing with water, condition the
Routine Use: Proteins can be eluted from PolyCAT A™ columns
with salt and/or pH gradients. The most useful range for cationexchange of proteins
is pH 6-7. Phosphate and Bis-tris are good buffers in this range. The higher
the pH, the weaker the retention.
Use ambient temperature (20-25°C), as this
polypeptide-based coating is more sensitive to elevated temperatures than are
other materials. Filter mobile phases and samples before use. Failure to do so
may cause the inlet frit to plug. This frit can be replaced.
Loading Capacity: The loading
capacity of a 4.6mm ID column is about 4 mg of protein/injection, depending on
the strength of the protein’s binding to the support.
Storage: 1) Overnight: 100% mobile phase A. 2)
Several days: Store in water. 3) Longer periods: Store in water in the refrigerator,
with the ends plugged. ACN can be added to the storage solvent (e.g.,
ACN:Water = 80:20) to retard microbial growth.
Column maintenance: After every 250
runs, invert the column and run it backwards overnight, at a low flow rate,
with 40 mM EDTA.2Na. Continue using the column in this inverted direction for
the next 250 samples, then repeat this treatment. If possible, open the inlet
and fill in any voids with bulk PolyCAT A™ after running 500
samples.
Minimize Iron in the System: This coating
chelates Fe+3, which ruins its performance. If chloride-containing
mobile phases are used regularly, passivate the column and the HPLC system
every 4 weeks with the 40 mM EDTA.2Na solution as described above.
NOTE: If the HPLC system has not been used for several
days (e.g., over a weekend), then Fe+3 ions tend to
accumulate in the fluid in the lines. When restarting the system, flush this
fluid to waste offline before diverting flow through the column.
Volatile Solvents: Below pH 4 the
coating loses its negative charge. Thus, peptides can be eluted by a gradient
to dilute acetic acid.
Miscellaneous applications:
1)Hemoglobins: These are well separated by PolyCAT
A™!
See the specific Application Note.
2)Growth Factors or Protein Variant separations:
Try an ammonium acetate gradient in 40% ACN. For separation of Asp- vs, isoAsp-
variants, try mobile phases at pH 4.2.
3)Antibodies: Human: try pH 6.4-7. Murine (=
mouse): try pH 7.2-8.0.
4)Chloride vs. acetate: Unlike chloride ion,
acetate does not corrode stainless steel. However, it is not transparent below
230 nm, and 10% more acetate is required to match the eluting power of
chloride.
5)Mixed-mode effects: When the mobile phase
contains over 60% organic solvent, then hydrophilic interactions will be superimposed
on the electrostatic effects. PolyCAT A™ can then resolve
many peptides that differ in polarity but not charge (e.g., methylation of a
Lys- residue). It may be necessary to use a gradient salt with good solubility
in org. solvents, such as sodium perchlorate or triethylamine phosphate (TEAP).
Cation-Exchange HPLC with Volatile Mobile Phases
This method works with PolyCAT A™, our weak
cation-exchange (WCX) material. A gradient to dilute acetic acid (HOAc) can uncharge
the carboxyl- groups on the surface, leading to the elution of retained
peptides. This gradient does not uncharge our strong cation-exchange (SCX)
material, PolySULFOETHYL A™. Peptides are reliably retained on a WCX material
only if they have three or more basic residues or two basic residues and a free
N-terminus. Thus, most tryptic peptides are not well-retained. This method also
works with nonpeptide basic solutes such as polyamines and aminoglycoside
antibiotics. Elution is generally in order of least to most basic, but there is
also some separation of sequence variants differing in nonbasic residues.
Adsorption: Apply the mixture to a PolyCAT A column
equilibrated with 10 mM ammonium acetate, pH 5-5.5. Binding capacity is approx.
4 mg. peptide for a 4.6-mm i.d. column.
Elution: Run a linear gradient to 15% aq. HOAc.
Some extremely basic peptides or polyamines have required as much as 30% HOAc
for elution.
Detection: Absorbance detection below 240 nm is
not possible with these mobile phases. Thus, absorbance detection is confined
to peptides with aromatic residues; 254 nm (for Phe-), 270 nm (for Tyr-), or
280 nm (for Trp-). Suitable alternatives include mass spectroscopy or an
evaporative light scattering detector (ELSD). Alternatively, just collect
fractions for bioassay after lyophilization or drying in a SpeedVac.
Notes:
1)Extremely basic solutes: If elution requires
over 20% HOAc, try PolyCAT A™ with 1000-Å pore diameter instead of 300-Å. This can
decrease by 3x the concentration of HOAc required.
2) Synthetic peptides: This is a convenient way to clean
up a crude synthetic product. Basic peptides are retained while deprotection
3) Unusually hydrophobic peptides: To promote solubility,
@ 20% organic solvent can be included in both mobile phases. Use of > 50%
organic solvent will result in hydrophilic interactions being superimposed on
the electrostatic effects.
4) Counterions: This method yields peptides with acetate
counterions. This is compatible with bioassays, unlike the trifluoroacetate counterion
frequently contributed by reversed-phase HPLC.
Acknowledgements to Mike Selsted @ U.C.-Irvine for
initial use of this technique.
Analysis of Hemoglobins using PolyCAT A™ columns
The following protocols were developed by Cheryl Rognerud
and Ching-Nan Ou at Texas Children’s Hospital (Houston):
For rapid screens or routine analysis use item#
3.54CT0510; 5μ, 1000Å.
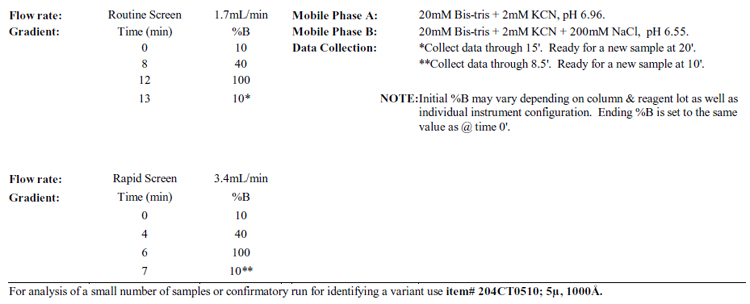
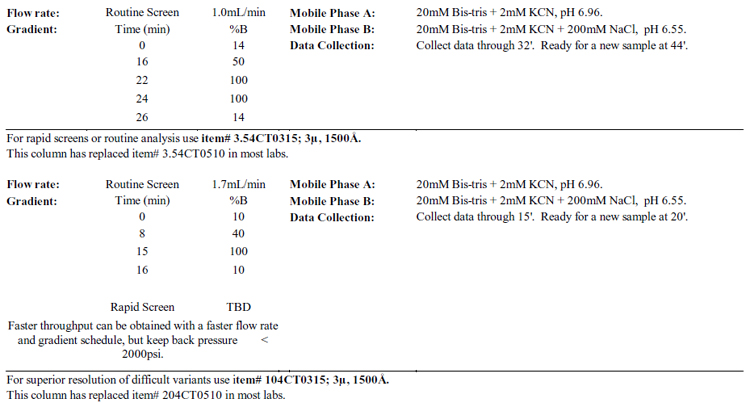

Notes: Do not degas the buffers with helium or vacuum!
Bypass any vacuum degasser in the system. Hemoglobin
tetramers exchange dimers in solution in a dynamic fashion. In the absence of
oxygen, this process is much slower.
Results: 1) Unnatural subunits are stabilized, resulting
in artificial peaks
2)
Artifacts can result from use of KCN or NaCN over 10 years old,
3)
Artifacts are also observed with poorly set automatic injector valves. Assess
this by using a manual injector valve if you suspect problems.
Loading Capacity: The loading
capacity of a 4.6mm ID column is about 4 mg of protein/injection, depending on
the strength of the protein’s binding to the support. If a PolyCAT A™ column
is to be used for preparative isolation of hemoglobins, then it is necessary to
use 5 – 10mM KCN in the sample solvent and mobile phases.
Storage: 1) Overnight: 100% mobile phase A. 2)
Several days: Store in water. 3) Longer periods: Store in water in the refrigerator,
with the ends plugged. ACN can be added to the storage solvent (e.g.,
ACN:Water = 80:20) to retard microbial growth.
Column maintenance: After every 250
runs, invert the column and run it backwards overnight, at a low flow rate,
with 40 mM EDTA.2Na. Continue using the column in this inverted direction for
the next 250 samples, and then repeat this treatment. If possible, open the
inlet and fill in any voids with bulk PolyCAT A™ after running 500
samples.
Preparation of Hemolyzates
1) Draw 1-2 ml of blood into a purple-capped tube (i.e.,
with EDTA). Alternatively, draw 100-200 μl with a fingerstick.
2) Washing away plasma proteins: Add 50 μl whole
blood to a 1.5-ml microcentrifuge tube with 1 ml isotonic saline (= 0.9% NaCl =
154 mM) and spin it for 30-60” @ 13,000 rpm. Discard supernatant.
3) Lysing the erythrocytes: Add water equal to
2-3x the volume of the packed cells. Let sit 5-10’ to lyse the erythrocytes.
The following are aids in lysing the cells: a) Vortexing; b) Including Triton
X-100 in the lysis solution; c) A freeze-thaw cycle.
4) Isolation of the hemolyzate: Centrifuge 5’ @
13,000 rpm to spin down the erythrocyte ghosts. The visible pellet should be grayish
and may occupy up to 1/3rd of the volume of the solution, with the clear red
lysate on top. This lysate is the hemolyzate.
NOTE: If the precipitate is red, then the erythrocytes
have not been lysed.
5) Preparation of samples for HPLC analysis: Add
20 μl lysate to 250 μl mobile phase A. Inject 8-10 μl per analysis with a 200 x
4.6-mm column or 4-8 μl for a 35 x 4.6-mm column. A partially filled loading
loop is acceptable. If this solution is to serve as a standard, then it can be
stored in a freezer in aliquots of 10-20 μl for future analysis.
Notes:
1) Smaller injections of more concentrated samples afford
better resolution than larger, more dilute samples.
2) Dot blot analyses from neonates: Prick the heel
of the neonate and blot a single drop of blood onto a piece of filter paper.
This can be mailed to the lab that has the PolyCAT A™ column. A circle
of the blood blot is cut out with a standard size paper puncher. This circle is
soaked for 2 hr. in 200 μl of Mobile Phase A containing 4 mM KCN (not 2 mM).
The resulting solution is centrifuged for 1’ to remove particulates and 25-50 μl
is injected for analysis; the optimum volumes depends on the percentage of Hb F
and the number of other peaks present.
3) Destroying labile glycated hemoglobins prior to A1c
analysis: Alternatives:
a)
In Step 2 above, incubate the washed erythrocytes in 0.9% NaCl for 1 hr. at
37°C before lysis.
b)
Same as a), but leave washed erythrocytes overnight at ambient temperature.
c)
Leave the washed erythrocytes overnight in mobile phase A (which lyses the
cells, see Step 5 above).
It usually isn’t necessary to destroy labile glycated
hemoglobins prior to analysis, since these are separated from stable A1c on PolyCAT
A™ columns
and elute in a “pre-A1c” peak.
주소
서울특별시 송파구 충미로 5 송파한화오벨리스크 C동 415호
연락처
전화번호 : 02-3012-9003 팩스번호 : 02-3012-9010
intertech9@naver.com
사업자등록번호
215-87-83507 대표이사 : 이홍근
